Abstract
Background: Recombinant porcine factor VIII (rpFVIII, susoctocog alfa, Obizur ®, Baxalta US Inc., a Takeda company, Lexington, MA, USA) is a recombinant, B-domain-deleted, porcine-sequence FVIII indicated for the treatment of bleeding episodes (BEs) in adults with acquired hemophilia A. Thirty to 40% of patients with congenital hemophilia develop inhibitors to human FVIII (hFVIII). rpFVIII has low cross-reactivity to anti-hFVIII antibodies and is predicted to provide functional FVIII activity in patients with congenital hemophilia A (CHA) with inhibitors (CHAWI).
Aims: To evaluate the efficacy and safety of rpFVIII in patients with CHAWI undergoing surgical and other invasive procedures.
Methods: This was a Phase 3, international, multicenter, single-arm, open-label study (NCT02895945). Eligible participants were male (aged 12-75 years) with severe (FVIII <1%) or moderately severe (FVIII ≤2%) CHA and inhibitors to hFVIII, who required elective surgical, dental, or other invasive procedures; those with anti-porcine FVIII (anti-pFVIII) >10 BU prior to surgery were excluded. Patients received a loading dose of rpFVIII 1-2 hours before surgery based on body weight, hematocrit, pFVIII inhibitor titer and type of surgery, followed by individualized dosing. The primary outcome was the proportion of all procedures with a good or excellent response (treatment success) on the global hemostatic efficacy assessment (GHEA) score, based on the full analysis set (FAS; surgeries with ≥1 available hemostatic assessment). The GHEA score comprises 3 individual surgeon/investigator-assessed ratings (excellent, good, fair, or none) summed to give the total GHEA score: intraoperative efficacy at the end of surgery (Day 0, GHEA1); postoperative efficacy on postoperative Day 1 (GHEA2); and perioperative efficacy up to 72 hours after the last postoperative dose of rpFVIII (GHEA3). Secondary efficacy outcomes included observed-versus-predicted operative blood loss, BEs, weight-adjusted rpFVIII administration, and adverse events (AEs). Analysis of safety was based on the safety analysis set (all patients who received any amount of rpFVIII).
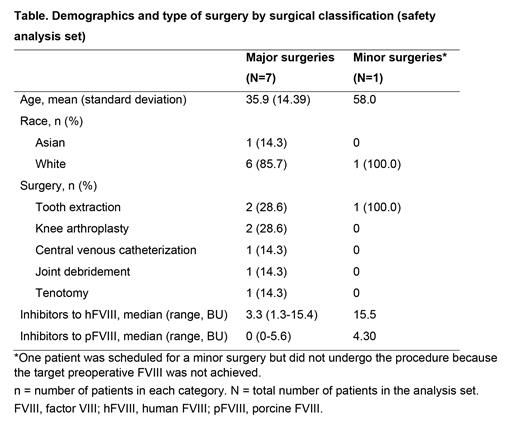
Results: Eight patients comprising the safety analysis set received ≥1 dose of rpFVIII from 8 sites in 5 countries (Table). Of these 8 patients, 7 underwent major surgeries and 1 was scheduled for a minor surgery but did not undergo the procedure because the target preoperative FVIII was not achieved. The FAS included the 7 patients with major surgeries. Of the 8 dosed patients, 5 completed the study. Six of 7 (85.7%; 95% confidence interval, 42.1-99.6%) surgeries achieved treatment success; 5 surgeries were rated excellent, and 1 was rated good. The mean (standard deviation) ratio of actual to expected average blood loss in the major surgeries was 0.97 (0.95) during the intraoperative period and 0.15 (0.22) during the postoperative period. Seven surgery-related BEs (FAS, intraoperative, n=1; postoperative, n=6) occurred in 3 patients during the study, with none requiring surgical intervention (n=2 moderate; n=3 major; n=2 missing severity). Overall, 6 of 8 patients experienced 17 AEs. Three patients had anamnestic reactions (increase in titer of ≥10 BU/mL from a measurable baseline) to hFVIII and pFVIII; 1 patient developed de novo pFVIII inhibitors; another 2 patients had anamnestic reactions to hFVIII and developed de novo pFVIII inhibitors. Eight serious adverse events (SAEs) were reported in 6 patients, of which 4 SAEs were considered related to rpFVIII (3 anti-pFVIII antibody positive; 1 anamnestic reaction to hFVIII and pFVIII). The unrelated remaining SAEs were anti-hFVIII antibody increased (n=2), bacterial infection (n=1) and haemarthrosis (n=1).
Conclusions: Six of 7 patients who received the study drug and underwent a surgical procedure achieved hemostasis with rpFVIII during the intraoperative and immediate postoperative period. Most patients developed de novo inhibitors or anamnestic reactions. Thus, good hemostasis can be achieved with rpFVIII within the first days after surgery; other bypassing agents should be used if longer clotting factor correction is required. The study was terminated early due to feasibility reasons and the risks of anamnestic reactions that outweigh the benefit in this study population.
Pfrepper: BMS: Honoraria; CSL Behring: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Shire: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer Healthcare: Membership on an entity's Board of Directors or advisory committees; Chugai: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; Fujimori: Research Funding; LeoPharma: Research Funding. Windyga: Baxalta: Honoraria, Research Funding; Novo Nordisk: Honoraria, Research Funding; Rigel Pharmaceuticals: Research Funding; F. Hoffmann-La Roche Ltd: Honoraria, Research Funding; Shire: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Alfasigma: Honoraria; Aspen: Honoraria; Bayer AG: Honoraria; Octapharma: Honoraria, Research Funding; Sanofi-Aventis: Honoraria, Research Funding; Sobi: Honoraria, Research Funding; Swixx BioPharma: Honoraria; Werfen: Honoraria; Alnylam Pharmaceuticals: Research Funding; Sanofi/Genzyme: Honoraria, Research Funding; Alexion: Honoraria; CSL Behring: Honoraria. Kavakli: Takeda: Consultancy, Other: Clinical Trial Support; Novo Nordisk A/S: Consultancy, Other: Clinical Trial Support; Roche: Consultancy, Other: Clinical Trial Support. Schutgens: Bayer: Research Funding; CSL Behring: Research Funding; Novo Nordisk: Research Funding; OctaPharma: Research Funding; Pfizer: Research Funding; Shire/Takeda: Research Funding. Baptista: Takeda Development Center Americas, Inc.: Current Employment; Takeda: Current equity holder in publicly-traded company. Badejo: Takeda Development Center Americas, Inc.: Current Employment; Takeda: Current equity holder in publicly-traded company. Ewenstein: Takeda Development Center Americas, Inc.: Current Employment; Takeda: Current equity holder in publicly-traded company. Jain: Takeda: Current equity holder in publicly-traded company; Takeda Development Center Americas, Inc.,: Current Employment.
This abstract includes discussion of the following investigational use of a drug: in the CHAWI study (NCT02895945), recombinant porcine factor VIII (susoctocog alfa) was not administered according to the dosing and administration information in the product label.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal